RAPIDO Wasn’t Designed to Answer This Question. So Why Are We Pretending It Did?
Toxicity analysis without planning data is just noise.
In the Red Journal this weekend, there was a paper looking at the RAPIDO trial and analyzing the toxicity differences between patients treated with IMRT/VMAT vs 3D. I had some thoughts.
As we all remember, the RAPIDO trial was a seminal study showing that TNT treatment strategy improved outcomes over the previous standard of pre-operative CRT +/- adjuvant chemotherapy (German style). The TNT arm utilized 25 Gy in 5 Fx followed by the entire course of chemotherapy, while the CRT arm treated to ~50 Gy concurrent with chemotherapy. Adjuvant chemotherapy was used at the discretion of the investigator or center. The overall study showed a benefit for TNT, which reduced disease-related treatment failure by lowering the risk of distant metastases. However, the TNT arm was associated with more local failures. Although TNT is becoming the standard of care for locally advanced rectal cancer, in the U.S. it remains common to use long-course CRT upfront, or to start with chemotherapy when the risk of distant disease is high.
So, this gets us to this new study, “Acute and late radiation-related toxicity after treatment of locally advanced rectal cancer with IMRT compared to 3D-CRT in the RAPIDO trial”.
I will have my friendly AI assistant summarize what they found:
This secondary analysis of the RAPIDO trial evaluated whether IMRT (including VMAT) reduced radiation-related toxicity compared to 3D-CRT in patients with locally advanced rectal cancer. The study found that IMRT did not lower rates of acute or late toxicity; in fact, IMRT was associated with more low-grade fatigue and nausea during short-course radiotherapy and chemotherapy (TNT arm), and higher rates of late sexual toxicity and overall late grade >1 toxicity in the chemoradiation (CRT) arm. Severe toxicities (grade ≥3), persistent toxicities, and treatment compliance were similar between groups, leading the authors to question whether IMRT provides meaningful clinical benefit in this setting.
When I see an outcome like this, I cannot just accept this at its face. “Low dose bath” was supposed to give us secondary malignancies galore. It didn’t. It was supposedly going to give us worse pulmonary outcomes. It didn’t. IMRT for other pelvic malignancies has improved toxicity outcomes. In this study, it did not. In fact, it showed worse outcomes and it doesn’t make much sense to me. I read the paper in detail and there are many flaws that need to be considered. Let’s go through them.
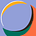
They did not contour OARs, track OAR doses or have OAR constraints. I don’t know why they did this, but if you don’t do any of those things, you are not doing IMRT. I have no heard of doing IMRT with no organs to spare and no volumetric dose constraints to meet. This is the original sin of this study and makes it very hard to take any of their findings seriously.
They didn’t have PTVs for 50% of the patients in either arm. Both 3D and IMRT/VMAT, if done correctly, have PTVs. We have no idea about coverage of the targets, or differences between volumes in patients treated with 3D and IMRT. Some patients had PTVs, but this was arbitrary and inconsistent—hardly enough for meaningful analysis.
We don’t have PTV margins defined for either group. Larger PTVs are associated with worse toxicity. This is why we use IGRT, motion management, SGRT, fiducials, and gated treatment—you get the point. They were allowed to do whatever they wanted with no direction from the protocol
The IMRT patients were much less likely to be in prone position or have a belly board. When I do rectal patients I use VMAT and I have patients on a belly board, positioned prone. This gives us the advantage of gravity pulling away the bowel from the pelvis, in addition to dose-shaping, reducing bowel dose substantially. It does not make sense to do one group mostly supine and the other group mostly prone. And for those who worry that prone positioning doesn’t set up as well— with routine IGRT, it is fine.
They did not select prospectively who would receive a boost and they did not track boost use by arm. Higher doses = higher toxicity. We have no idea which group had a boost and why they received one.
The study started many years ago (2011) and IMRT/VMAT planning has improved over time. Many people feel that VMAT and SIB would allow for better conformality of plans and that this could also improve outcomes. I don’t know that we have data for this, but I do also treat with VMAT and SIB, because of this theoretical advantage.
The patients treated with IMRT were not randomly selected. This being the case, it is possible that investigators treated their frail / sicker patients with IMRT. Given the different financial incentives in Europe, this seems plausible. Investigators—there and here—often view IMRT/VMAT as superior, and may have chosen it for frailer patients, skewing outcomes.
The main finding was increased nausea and fatigue in IMRT patients. There’s no plausible mechanism for this, and it hasn’t been observed in other anatomic sites when comparing 3D and IMRT. This to me seems like a spurious outcome.
This is a weak analysis that I wouldn’t use to guide treatment decisions—and certainly not for policy or payer determinations. This is not strong data to support 3D over IMRT. I will concede it is also not supportive of IMRT over 3D, but the outcome flies in the face of what we know about radiation oncology. And, if you are wondering how we would study this for rectal cancer, here is what I would consider:
The prospective data isn’t there. I will concede that. We had one study that had significant flaws (RTOG 0822), using chemotherapy we don’t use (cape + ox) and there was no control arm. They only examined acute toxicity, but late toxicity is just as important—especially in this setting, as many patients are cured. But, yes, this was a negative trial.
There is a retrospective study from Mayo Clinic by Samuelian, et. al that does support what I see in real life - less acute toxicity:
They found that IMRT significantly reduced acute GI toxicity in rectal cancer patients compared to 3D-CRT, with lower rates of diarrhea and enteritis. Pathologic response and postoperative outcomes were similar between groups, supporting IMRT as a better-tolerated option without compromising efficacy.
The other factor is that these patients are undergoing concurrent chemotherapy and so myelosuppression from RT is a concern. With IMRT, we can reduce bone marrow dose and this has been shown to lower toxicity. The studies that have been done do not look at this, although the gyn study, RTOG 0418, did show benefit.
In residency, my institution treated nearly all rectal cancer patients with IMRT. In 15 years of practice, I’ve treated 100s of rectal cancers, but only a handful with 3D. Frankly, I would find it hard to go back to 3D based on this study or RTOG 0822. When the evidence is weak, we have to rely on clinical intuition and pragmatism. I am not holding my breath for a comparative effectiveness study - I feel that there are better uses of our time and energy. However, if they would like to do one, they should consider what I have suggested.
Happy Mother’s Day to my mom readers. Ours was lovely. My kids have literally the best mom ever!
Love you all,
Sim